The first half of the first lecture is taken up with course organization matters. The second half is where we start introducing physics ideas.
Physicists have a great job: we find an interesting system (shiny object) to study, then we poke it and see what happens. What most fascinates us is how properties of the system change over time and across space. For instance, the patterns of winds on planet Earth. There are three principal aspects of the modern physics enterprise: measurement (experiment); mathematical modelling (theory); and computing, on personal computers or supercomputers. Physics as a structure is a bit like a three-legged stool, in that it requires all three legs to be structurally sound and to work together harmoniously. Different types of physicists specialize in different areas.
In my own work, I do theoretical physics research using pen and paper and on personal computers, performing a lot of symbolic algebra manipulations and some numerical computations as needed. My specific area of interest is how to marry quantum mechanics with gravity (my favourite force in the universe!) using a modern conceptual framework known as string theory.
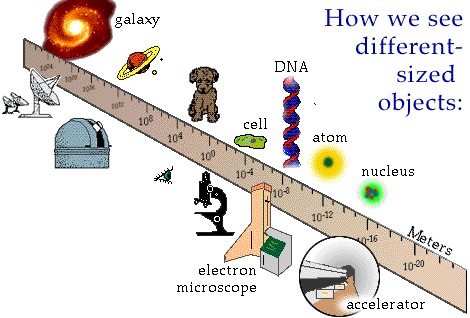
Physicists start analyzing a system by first determining its size. We do this for a very simple reason: size is really important to what tools a physicist should use to poke it and measure its response. For instance, if you wanted to do brain surgery, you would pick precision brain surgery instruments with delicate tips, rather than an axe, a chainsaw, and an industrial drill press. Picking the right-sized tool for the job in physics is just as crucial: it can mean the difference between making an original discovery and not making one. Precision is important to being able to discern patterns.
Because we are interested in physical principles operating over a wide variety of length scales, physicists need to be able to discuss ultra-teeny microscopically small numbers with lots of decimals -- and huge macroscopically large numbers with loads of zeroes -- and somehow keep our paper consumption to a minimum! It turns out that there is a really smart way to do this: use a concept called powers of 10. The basic idea of powers of 10 is that every time we zoom in/out by a factor of ten, we lose/gain one in the power of 10. We also define $1=10^0$. Let us do a couple of examples to illustrate this. Suppose that we zoom in from one metre by a power of ten. That would take $10^0$m to $10^{-1}$m, i.e., one tenth of a metre. Or suppose that we zoomed out by three powers of ten, in other words by a factor of a thousand ($10\times 10\times 10$). That would take $1$m$=10^0$m to $10^3$m, commonly known as $1$km. In the SI metric system, zooming in or out by factors of a thousand is so handy that those powers of ten get their own SI prefixes.
The powers of 10 notation really comes into its own when we are talking about extremely small or extremely large numbers. The smallest distance ever measured by humans is about $10^{-18}$m. The very edge of the visible known universe is about $10^{+25}$m away. If we did not use powers of 10 to write them, those two numbers would be $0.000 000 000 000 000 001$m and $10 000 000 000 000 000 000 000 000$m respectively. As you can see, both are pretty cumbersome to write longhand. The powers of 10 notation is compact and powerful.
Powers of 10 are illustrated very nicely on the powersof10.com web site, which is worth exploring. In particular, I recommend watching the (8-minute) original Powers of Ten movie by Charles and Ray Eames on YouTube. It is really helpful to watch, to help you get your head around how big the universe is and how small subatomic particles are. Here are a few samples of what the world looks like at bigger or smaller length scales than ourselves (images from powersof10.com):-
NASA has a beautiful collection of pictures of Earth from space which you may want to peruse.
Ernest Rutherford (a.k.a. Lord Rutherford of Nelson) was a New Zealander, and is honoured today by having his image on the back of the NZD100 bill. He is the only really famous alum of my undergraduate alma mater, the University of Canterbury. He is famous for work he did in Canada and Britain, including splitting the atom
in 1911. His brilliant idea was to fire little missiles called $\alpha$-particles at Gold atoms in a thin foil, to figure out what they were made of. $\alpha$-particles are helium nuclei and are produced in one type of nuclear fission reactions. Here is a schematic diagram of Rutherford's apparatus; the actual experiments were carried out by Hans Geiger and Ernest Marsden.
Rutherford was expecting his alpha particles to get deflected either not at all only a little bit as they went through the atom, which physicists of the day thought was constituted much like a plum pudding. Their conception was that positively and negatively charged particles within the atom would be roughly evenly distributed throughout, so the $\alpha$-particles would not get a particularly bumpy ride through the atom. Rutherford expected his fluorescent screen to show flashes indicating either no deflection or minor deflections of alpha-particles, with a soft-looking distribution. But instead, he got a surprise so unexpected he said it was like artillery shells bouncing back off tissue paper! Yes, there were a lot of undeflected particles, but there were also a few very large deflections along with the smaller ones. The results gave the Rutherford team a harder
distribution than expected. Rutherford realized that the large deflections imply that there is an extra-hard little nuggetty structure deep inside the atom that bounces the impinging $\alpha$-particles off at cock-eyed angles. Based on Rutherford's team's work, we now know that the positive charge of the atom is concentrated in a very dense, hard, small region about 100,000 times smaller than the width of the atom, known as the nucleus, while the negative charge of the atom is carried by a fluffy cloud of electrons surrounding the nucleus. The radius of an atomic electron cloud is of the order of $10^{-10}$m, also known as an Angstrom, while the nuclear radius is about $10^{-15}$m in size.
Have you ever wondered why the atomic nucleus does not explode? Inside the nucleus there are positively charged protons and electrically neutral neutrons. The problem is, if you have an atom of (say) Carbon, which has 6 protons, then the protons in the nucleus are all repelling each other electrically, because like charges repel. This is just static electricity like happens to your hair after you take your hat off in winter. Now, since the nuclei in our bodies are not constantly exploding, there must be another force so strong that it overwhelms the electric repulsion of the protons in the nucleus and binds them together tightly inside the tiny nucleus in a stable way. This other powerful force is called the strong nuclear (or colour
) force.
Particle physicists did not stop at Rutherford's experiment: they kept developing techniques, building better accelerators, and making new discoveries. One of the Nobel Prize worthy discoveries happened at SLAC, a big particle accelerator lab near Stanford University in California, back in the hippy era. Physicists there had the bright idea of firing electrons into protons and neutrons -- and they discovered substructure! Each proton and neutron has three little nuggetty bits inside called quarks, bound together strongly by gluons. We will say quite a lot more about quarks and the colour force next week.
Students interested in taking things to the next level may wish to study the famous Feynman Lectures on Physics.